proprio AFO.
The concept of a 3D-printed orthoses and sensorimotor insoles
Concept
3D-printed AFO easily configured and with seamless integration of proprio SOLE.
proprio is the market-leading and established therapy concept for sensorimotor foot orthoses from Springer. The extended proprio concept offers you the seamless integration of proprio SOLE and proprio AFO. Perfectly adapted to the work processes in your company.
We show you how to make the best 3D orthoses easily and intuitively.
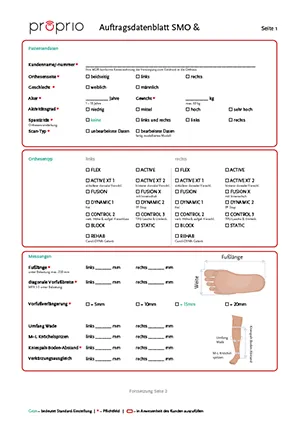
As an orthopedic technician, you have complete freedom when planning your patient care thanks to the wide range of models. Using our digital tools, you can model the insole and configure the orthoses in a simple, safe and reproducible workflow.
What you need:
Models
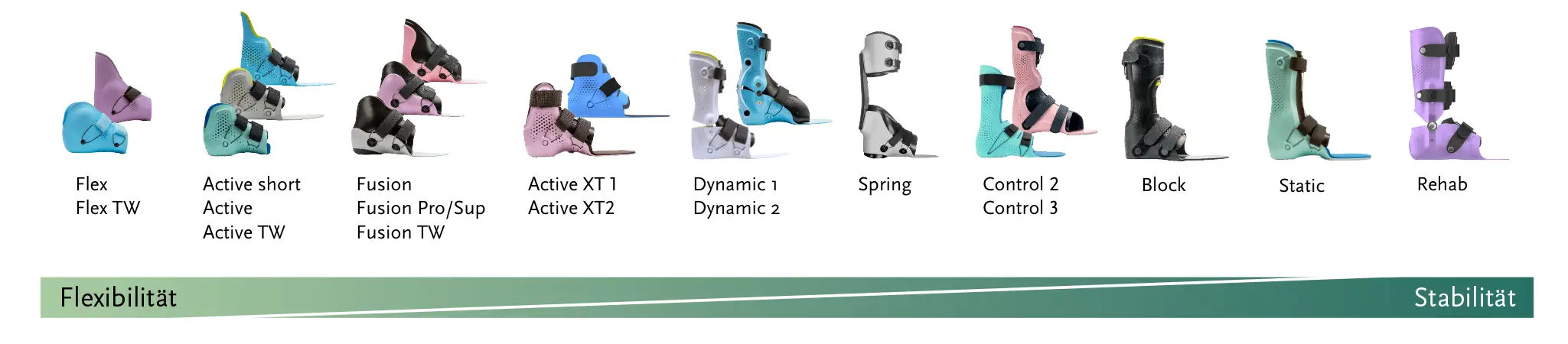
Flexible to stable configuration
The ultra-light but dimensionally stable and break-proof materials as well as the convincing design of the proprio AFO guarantee you satisfied patients.
As an orthopedic technician, you have complete freedom when planning your patient care thanks to the wide range of models. Using our digital tools, you can model the insole and configure the orthoses in a simple, safe and reproducible workflow.
These colours can be freely combined for the orthosis and upholstery:
Orthosis:
Upholstery:
CONFIGURATION
A simple and intuitive workflow.
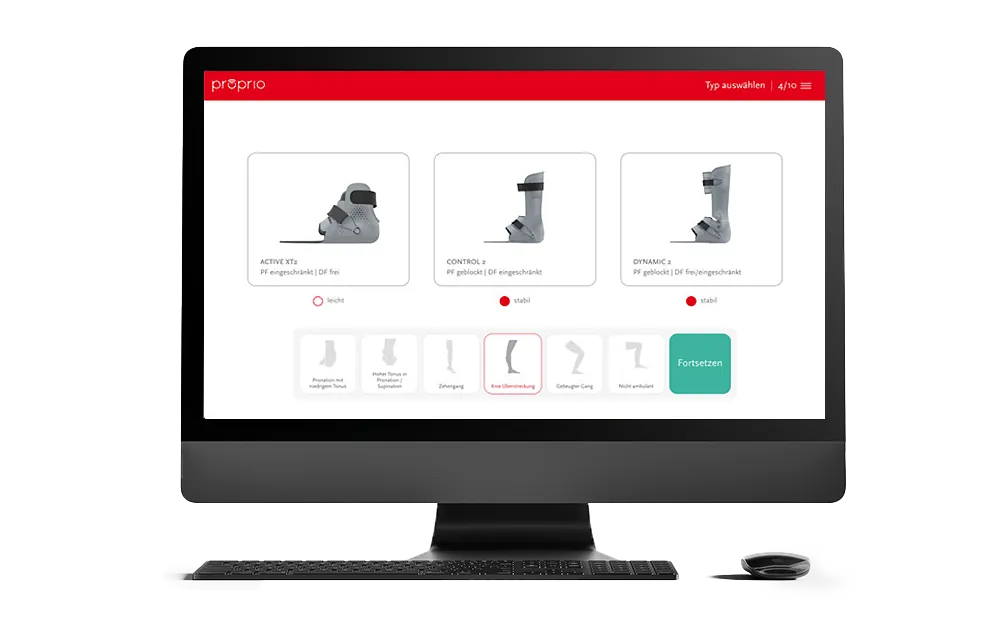



You want to start?
proprio gives orthopaedic specialists a quick and easy entry into the world of 3D-printed orthoses.
Thanks to digital training, a free configuration process and precise service production, you benefit from the advantages of digital production with the proprio AFO.
Make a risk-free start to the digitalisation of your Workshop.
The starting signal
Close to the customer
Technical support and guidance in the first implementation phase by master orthopaedic technician Axel Ruppert.
MDR-compliant
proprio AFO are MDR-compliant. The end product remains a customised product made by your company. You add your declaration as usual. Your product labelling is also printed on the product. The manufacturer's declaration of conformity and instructions for use ensure a safe, MDR-compliant framework. In the event of an audit, you can refer to these for the majority of the technical documentation and clinical validation.